Bioinformatics in the Preclinical Stage
- 7th October 2020
- Posted by: Breige McBride
- Categories: Articles, Bioinformatics, Gene Expression Analysis
Bioinformatics has a significant role to play in the preclinical stage of the drug development process and beyond into the clinical phases of drug development. Often overlooked, robust data analysis can help with answering key questions and reducing wet lab work to hasten the drug discovery process. Below we explore some of the ways that bioinformatics can support the drug discovery process at the preclinical stage.
Bioinformatics in Target & Lead Selection
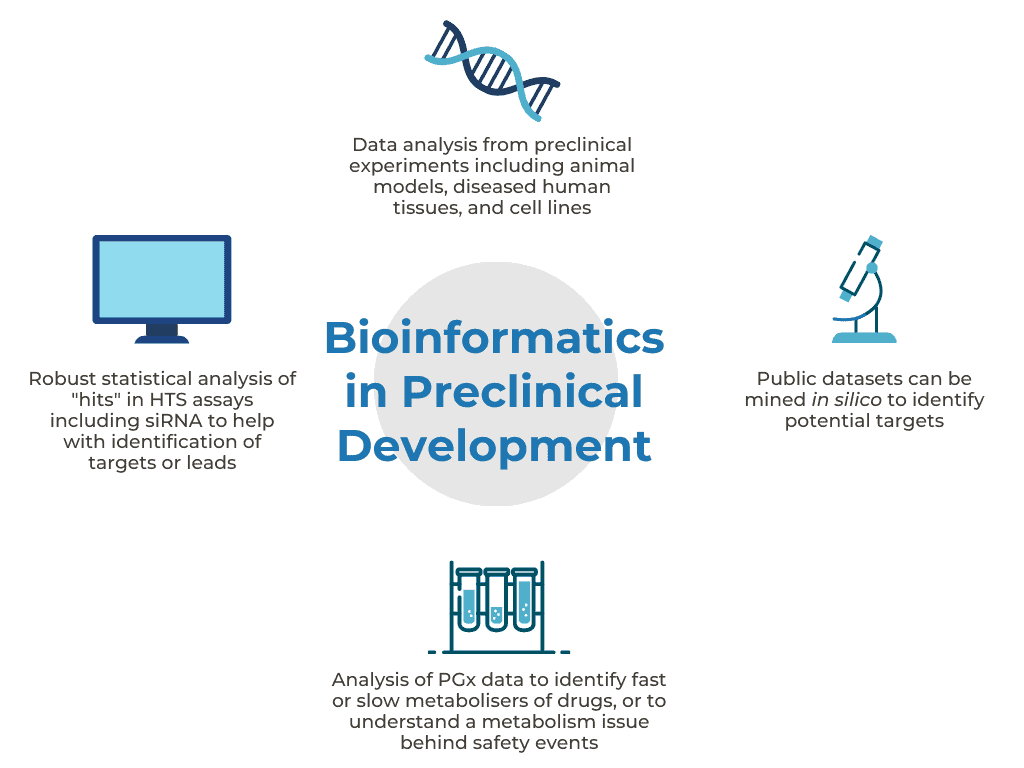
Bioinformatics can support your research from the early stages on the road to drug discovery. Robust analyses of data generated from High-Throughput Screens (HTS), or from preclinical experiments using animal models, diseased human tissues, and cell lines, can help with the identification of targets or leads. Public datasets can also be mined in silico to identify potential targets. Additionally, lead selection can be undertaken via a comparative analysis approach involving multiple assets, multiple concentrations and/or multiple formulations.
Once identified, targets and leads need to be validated. In silico comparisons of preclinical results with publicly available public data can substantiate their validation. Target validation can also be supported by vigorous analyses of data generated from CRISPR screens, or gene expression data generated from models in which the target has been knocked-out or knocked-down. Likewise, lead validation can be established by comparing gene expression data generated from untreated models and models treated with the identified lead. This comparison gives insights into the lead mode of action.
Bioinformatics in Formal Preclinical & Clinical Development
The aim of the preclinical and clinical development stages is to demonstrate that the validated drug has beneficial therapeutic effects in at least one indication, within accepted safety limits. Bioinformatics can help with achieving this objective by, for example, identifying off-target effects, on-target toxicity or analysing early tox readouts. Bioinformatics can also help in assessing changes over time in patients, through longitudinal biomarker analyses.
Additionally, statistical modelling of pharmacokinetics data and relating exposure to pharmacodynamic markers of interest are very useful to understand the potency and efficacy of the validated lead. Analyses of pharmacogenomics (PGx) data enable the identification of fast or slow metabolisers of drugs. These PGx analyses can help to understand a metabolism issue behind a safety event that occurred in a patient.
In summary, bioinformatics is an essential tool in the toolbox for drug discovery which can help you all along the drug development process to build the full picture of your new drug. Particularly at the preclinical stage, bioinformatics can help to gain more insight into the method of action of a therapeutic and highlight any immediate safety concerns meaning you can fail earlier and save money. Furthermore, the information gathered at this stage through the analysis can offer guidance for future clinical trials.
Bioinformatics at Fios Genomics
As a bioinformatics service provider, Fios can assist you all along the drug discovery process. We offer robust analyses endorsed by statistical tests and focused on the biological interpretations of the outcomes. Find out more about our published clinical-stage work on our publications page.
Read more on the preclinical drug development process in our previous blog post.
Leave a Reply
You must be logged in to post a comment.

