Collaboration between G1 Therapeutics and Fios Genomics for Lerociclib and Trilaciclib studies presented at ESMO, AACR and SABCS
- 18th January 2019
- Posted by: Breige McBride
- Categories: Articles, Bioinformatics, Gene Expression Analysis
Often, the work we undertake for clients is featured in articles or posters presented at conferences. Analyses we performed in 2018 on behalf of G1 Therapeutics were featured in four of their posters.
G1 Therapeutics is a clinical-stage biopharmaceutical company based in North Carolina. They are focused on the discovery, development and delivery of innovative therapies that improve the lives of those affected by cancer. They have a pipeline of drug candidates over a number of oncology indications, and regularly release publications surrounding therapies in their pipeline.
AACR Annual Meeting – G1T38
The first poster, ‘The CDK4/6 inhibitor, G1T38, enhances response to targeted therapies in preclinical models of non-small cell lung cancer’, was presented at the AACR Annual Meeting in April 2018.
Lerociclib (G1T38) is a small-molecule cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitor that is currently in development. CDK4/6 are key therapeutic targets and the use of inhibitors against these has applications for multiple tumour types1. Clinical data has shown that lerociclib in combination with fulvestrant in ER+ breast cancer can be dosed continuously without causing dose limiting neutropenia.
As this mechanism has potential to treat multiple cancer types, G1 Therapeutics is also evaluating the utility of lerociclib in non-small cell lung cancer (NSCLC). NSCLC is one tumour type that is predominately CDK4/6 dependent. NSCLC accounts for 80-85% of lung cancers, meaning that new therapies are needed. In NSCLC, a number of tumour development pathways use CDK4/6, meaning that lerociclib acting as an inhibitor can be effective in reducing tumour growth1.
The data analysed by Fios centred on tumour growth inhibition (TGI). After G1 Therapeutics evaluated single agent efficacy in ~50 NSCLC PDX models, Fios evaluated the optimal TGI cutoff to determine the models that are responders or non-responders. In previous work, the response definition of tumour regression was established according to NCI standards, where antitumor activity can be defined by a minimum value of 58% TGI2.
The trial found that lerociclib enhanced the anti-proliferative effect of inhibitors and demonstrated single-agent activity in models of NSCLC. Used as part of a combination therapy, lerociclib enhanced efficacy1.
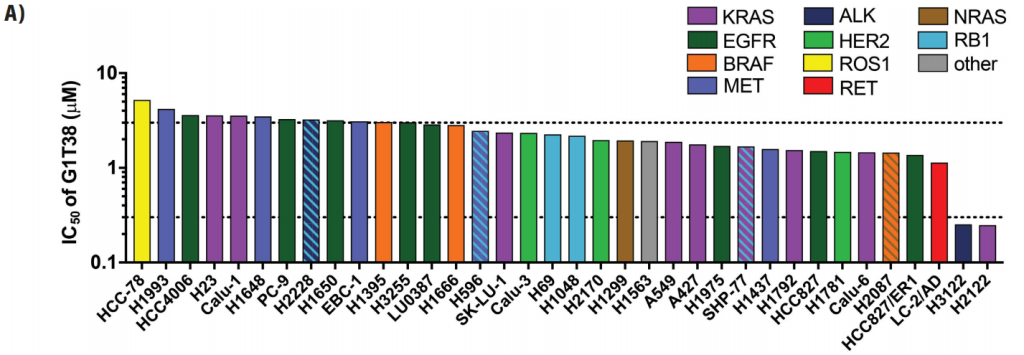
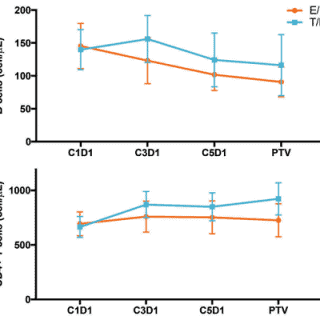
Figure 3A: Lung cancer cell lines (n=36) harboring known oncogenic mutations were screened for sensitivity to G1T38 alone or in combination with relevant targeted kinase inhibitors (Crown Bioscience, Taicang, China). (A) Relative IC50 values from single-agent G1T38 treatments were calculated using a 2x doubling time cell proliferation assay (minimum 3 days).1
AACR Annual Meeting – Trilaciclib
The second poster, ‘Transient exposure to trilaciclib, a CDK4/6 inhibitor, modulates gene expression in tumor immune infiltrates and promotes a pro-inflammatory tumor microenvironment’ was also presented at AACR 2018.
Trilaciclib (G1T28) is a first-in-class myelopreservation and CDK4/6 inhibitor therapy designed to improve outcomes of patients who receive chemotherapy by preserving hematopoietic stem and progenitor cell (HSPC) and immune system function3.
Only a minority of patients respond to immune checkpoint inhibitors (ICIs) by themselves. To increase the response rate of ICIs, one method is to combine them with chemotherapy. This causes immunogenic cell death that can help to ‘prime’ the immune system, however this can cause immunosuppression. The addition of trilaciclib to chemotherapy/ICI combinations was tested based on its ability to preserve HSPCs and enhance immune system function during chemotherapy3.
Mouse models were treated with trilaciclib and control treatments and subsequently profiled for gene expression levels using the PanCancer Immune Profiling Panel (NanoString). Fios performed the analysis of the gene expression data including normalisation, quality control, statistical hypothesis testing and pathway analysis. The identified genes and pathways were visualized using clustered heatmaps and barcharts.
In preclinical animal models, trilaciclib induces a transient G1 cell cycle arrest of HSPCs.
Administration of trilaciclib prior to chemotherapy results in improved recovery of complete blood counts, preservation of the immune system, maintenance of long-term bone marrow function, prevention of myeloid skewing, and enhancement of antitumor efficacy3.
A Phase 2 trial for patients with SCLC demonstrated that trilaciclib reduced clinically relevant consequences of chemotherapy-induced myelosuppression compared with a placebo.
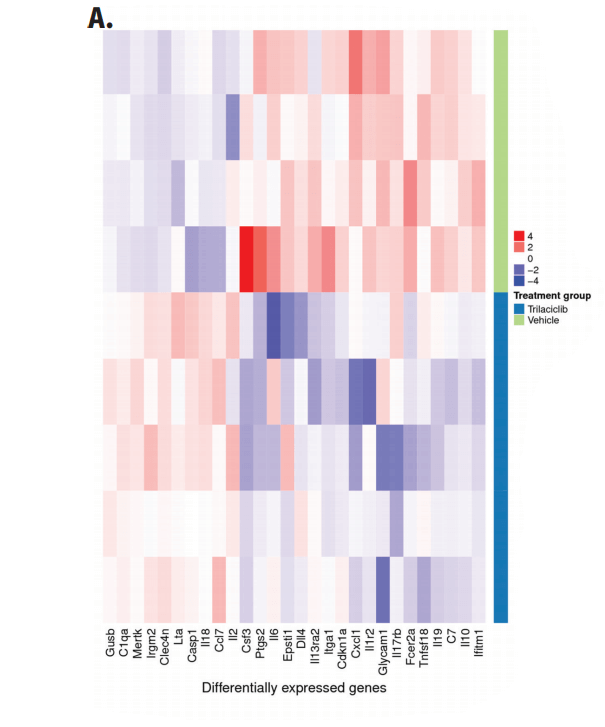
Figure 3A: MC38 tumor-bearing mice were treated with two weekly doses of trilaciclib (100 mg/kg) and tumors were harvested 24 hours after the last dose for analysis (n=5 per group). Gene expression profiling was performed using the PanCancer Immune Profiling Panel (NanoString). Normalized and Log2 transformed expression values were used for identification of differentially expressed genes and Gene Ontology (GO) term enrichment analysis. A. Heatmap displaying genes that were significantly upregulated (red) and downregulated (blue) in tumors after trilaciclib treatment. Twenty-eight differentially expressed genes were identified, defined using a p-value < 0.05 and absolute fold-change ≥ 1.3.3
ESMO Congress
The third poster we were featured on, ‘Trilaciclib preserves and enhances immune system function in extensive-stage small cell lung cancer (SCLC) patients receiving first-line chemotherapy’, was presented at the ESMO 2018 Congress in October.
Currently, there are no therapies on the market that exist to preserve HSPC and immune system function for those undergoing chemotherapy. Trilaciclib is administered before chemotherapy and it is currently being evaluated in Phase 2 randomised trials4.
SCLC was chosen to evaluate trilaciclib’s effect on HSPC and the immune system because a common chemotherapy regimen for 1st-line SCLC (carboplatin + etoposide) results in a high degree of myelotoxicity and there is a need for improved patient outcomes. Results were collected from whole blood samples of patients who have received 4-6 cycles of chemotherapy4.
The analyses were completed at Fios. Fios performed quality control and harmonisation of the flow-cytometry data, followed by linear mixed-effect modelling of selected cell subpopulations. The models were fitted on the longitudinal data for each of the cell types, characterising cellular abundance, either absolute or relative. The patient was considered as a random effect, while time (in weeks), treatment (Trilaciclib or Placebo) and their interaction were modelled as fixed effects and visualised using line plots.
The study found that chemotherapy decreased the number of circulating B cells over time but did not affect the number of other lymphocyte populations in the blood. The addition of trilaciclib preserved the number of circulating B cells while increasing the number of circulating CD8+ and CD4+ T cells4.
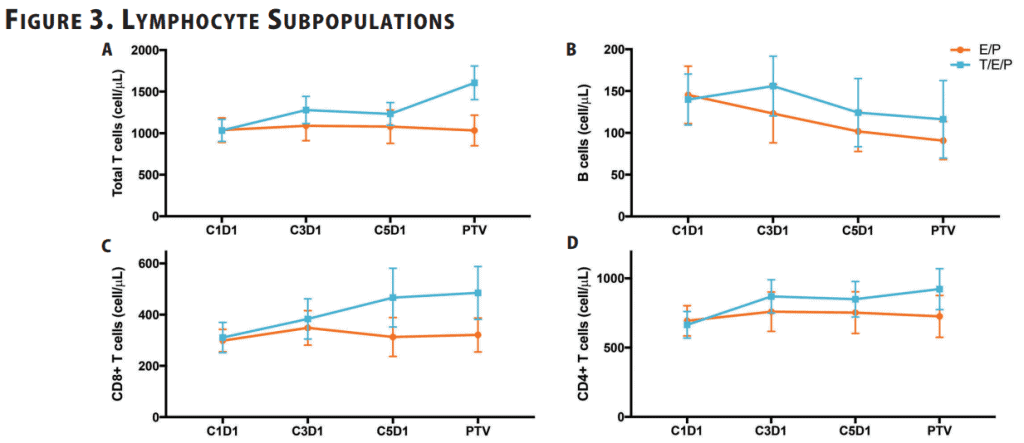
Figure 3: Mean absolute cell count for (A) T cells (CD3+), (B) B cells (CD19+), (C) CD8+ T cells, (D) CD4+ T cells in whole blood was analyzed by flow cytometry at the indicated time points. Error bars represent 95% CI.4
San Antonio Breast Cancer Symposium
The final poster, ‘Trilaciclib, a CDK4/6 inhibitor, dosed with gemcitabine, carboplatin in metastatic triple negative breast cancer (mTNBC) patients: preliminary phase 2 results’, was presented at the San Antonio Breast Cancer Symposium in December.
Chemotherapy regimens used for mTNBC are cytotoxic and treatment can be limited by myelotoxicities. G1 Therapeutics evaluated the effects of trilaciclib on a gemcitabine and carboplatin (GC) regimen for the treatment of mTNBC5.
Trilaciclib was administered immediately prior to gemcitabine and carboplatin (GC) chemotherapy. The addition of trilaciclib to GC treatment decreased the frequency of occurrence of myelosuppression events and their consequences compared to when patients were treated with GC by itself. Of the treatment emergent adverse events, such as fatigue and nausea, that arose during the study, most were attributable to the chemotherapy administered. The combination of trilaciclib and GC showed a significant decrease in the rate of occurrence of major adverse hematologic events compared to GC chemotherapy alone. Major adverse hematologic events included severe neutropenia and the need for red blood cell transfusions5.
The analysis that Fios completed for this trial surrounded the flow cytometry data for patients who received three or more cycle of GC chemotherapy. Fios performed quality control and harmonisation of the flow-cytometry data, including exploratory analysis. Trends over time for selected cell subpopulations were visualised over the longitudinal series to indicate time-dependent changes.
Overall, patients who received GC chemotherapy in conjunction with trilaciclib had a longer progression-free survival compared with just GC chemotherapy alone. The data from this study suggest that co-administering trilaciclib with the standard chemotherapy treatment can preserve multiple arms of the host immune system, and potentially enhance patients’ CD8+ T cell function5.
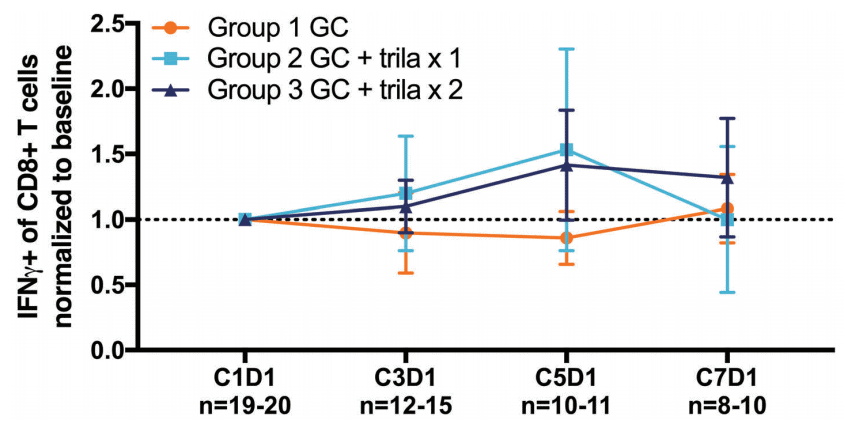
Figure 6: Normalized mean frequency of IFNg+ population of CD8+ T cells after ex vivo stimulation (IFNg+IL-17A-(CD3+CD8+)). Cell populations in whole blood were analyzed by flow cytometry at the indicated time points at Covance Central Laboratories. Analyses were completed at Fios Genomics and included only patients who received 3+ cycles of chemotherapy with statistical outliers excluded. Error bars represent 95% confidence interval (CI).5
More information
For more information on trilaciclib, lerociclib and G1 Therapeutics, visit the G1 website.
Visit our publications and posters pages to see more examples of the work we provide for our clients.
If you would like to talk to us about a study you are planning or analyses you would like carried out, send us an email or fill out our contact form today for a free quote.
References
2 – NCI Standards
REQUEST A SAMPLE REPORT
Want to know more about our data solutions? Fill the form to receive a sample report.
Leave a Reply
You must be logged in to post a comment.

