Preclinical Phase Of The Drug Development Process – An Overview
The drug development process has several stages which can take decades to complete. Understanding the stages novel drugs must go through is essential to understand how new therapeutics and vaccines enter the market. Below we take a look at the steps involved in the preclinical phase of drug development.
Background
The pharmaceutical industry as an entity is relatively young. Initially, pharmaceutical companies focused on curing diseases rather than treating them. However, thanks to the advent of systematic drug development, active ingredients for therapeutics came into the picture and companies started to manufacture them at scale. As a result, pharmaceutical product development accelerated, allowing treatment for chronic diseases or those without a cure.
Nowadays, drug development is a hugely expensive business. In fact, estimates put the cost of developing a drug, from synthesising the compound in the lab to delivering it to patients, at between £350M and £2B. Obviously, this is not a small investment even at the lower end of the scale. What’s more, this expensive process can take decades. And even then, only around 4% of the drugs launched into the marketplace make a return on their initial investment.
Drug Discovery
Much of the boom in drug discovery and pharmaceutical product development is due to advances in chemistry and compound screening development. Combinatorial chemistry is responsible for the generation of vast compound libraries that can be screened.
These libraries are searchable with High-throughput screening (HTS). This is an automated process to look for hits, where each screen is an assay for a particular target, such as a protein. As the name suggests, HTS generates a vast amount of data and can collect millions of data points. The analysis at this stage is often to detect an increase or decrease from the baseline normal for the target. For protein and biomarker targets, expression can be altered and up- or down-regulated. The ‘hit’ gives a starting point for which compounds to focus on.
HTS is, however, a high effort, low yield activity in the preclinical phase. This is because researchers use it to test thousands of compounds against the targets to find potentially hundreds of candidates, with no statement on their efficacy. Thus pharmaceutical companies create pipelines which are built around the drugs created rather than pipelines for a specific target disease. Often, researchers identify surrogate markers for an illness, such as specific but unrelated biomarkers, instead of biologically relevant ones.
Consequently, HTS use has decreased in favour of more rational drug design. This includes knowledge of biological targets, so drugs can be designed in the preclinical phase with specific biomarkers in mind.
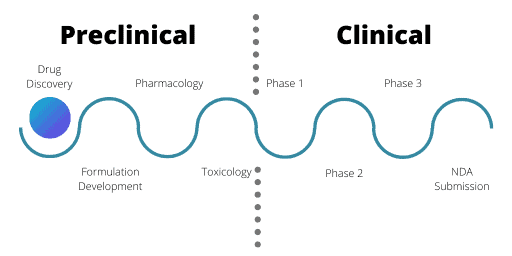
The Drug Development Cycle
There are two broad sections to the drug development cycle: the preclinical phase and the clinical phase.
Generally, biotechnological companies specialise in the preclinical phase and develop numerous drug candidates, running preliminary studies to test viability. Once a compound has reached a specific stage, they will partner with pharmaceutical companies to develop the candidates further or sell the assets on.
Preclinical Development
Once researchers have identified a potential drug via the research and development stages, preclinical testing can occur. For preclinical and clinical testing, agencies worldwide regulate and monitor the industry. For the UK it is the Medicines and Healthcare Products Regulatory Agency, whereas the USA has the Food and Drug Administration.
These agencies process Investigational New Drug (IND) applications which, once approved, allow clinical trials to proceed. There are three parts in IND applications: formulation development, pharmacology, and toxicology.
Make the most of your preclinical research
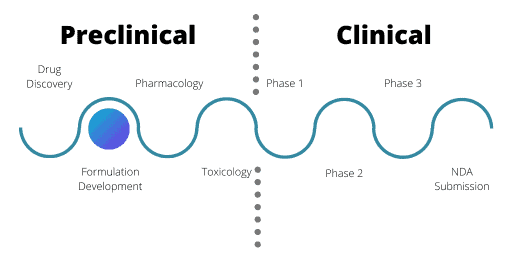
Formulation Development In Preclinical Phase
Formulation development assesses the best way to prepare a drug in the preclinical phase for its intended clinical use in patients. Factors such as solubility, frequency and mode of administration, stability of the formula, and palatability are all assessed. This takes into consideration where the disease or problem occurs, such as the sinuses or tumour cells.
Developing the formula is only half the story – manufacturing is also vital here. At the R&D stage, a drug may only be produced in milligram quantities, but for development, kilograms are needed. Manufacturing needs to adhere to Good Manufacturing Procedures and must test every batch of the potential drug to ensure the purity, quality and potency.
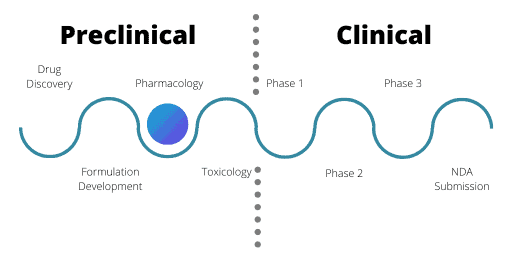
Pharmacology
The pharmacology stage assesses the safety of a drug as well as its ADME – Absorption; Distribution; Metabolism; and Excretion. ADME is the backbone of pharmacology.
ADME
Absorption concerns the bioavailability of the drug once administered. Before most drugs can achieve their goal, the bloodstream carries them away. The bioavailability of the drug measures the fraction of an administered drug dose that reaches the target system, while also measuring the uptake in the target cells and organs.
Once the body has absorbed a drug, the latter distributes from one part of the body to another.
Metabolism studies the metabolites produced by the drug’s breakdown. It then evaluates whether they are active or harmful, and the breakdown’s location in the body.
Finally, excretion looks at how the drugs and the metabolites produced exit the body.
While ADME is not a regulatory requirement for a first-time, in man (FTIM) study, having conducted it helps improve the quality of the safety information and gives further information to pharmacology and toxicology studies.
Safety
The safety assessment of a drug monitors both pharmacodynamic (PD) and pharmacokinetic (PK) interactions. PD interactions are where the drug administered can affect the actions of another specified drug without affecting its concentration, such as warfarin and antibiotics used in tandem. PK interactions are where the drug administered can affect the actions of another specified drug by affecting its concentration or that of its metabolites, such as the relationship between alcohol and paracetamol.
Safety plays a huge part in the preclinical stage of pharmaceutical product development: researchers must test any drugs vying for IND status extensively before allowing human testing. Researchers can use computer modelling and simulations to an extent, however, they still require animal models to assess safety. This is because they will assess the pharmacodynamics of the drug on a number of the major body systems such as:
- the cardiovascular and respiratory systems
- renal functionality and intestinal transit
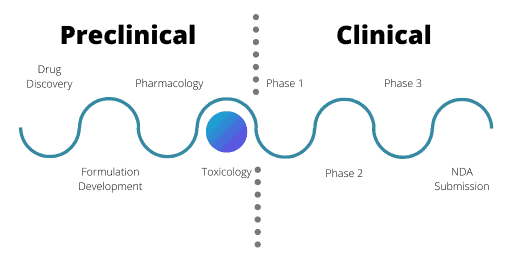
Toxicology In The Preclinical Phase
Since researchers need to conduct IND tests in animal models, they must conduct studies in two species. This part of the testing is highly regulated. Researchers must use one species that is non-rodent and their use of primates as an animal model is heavily restricted.
The toxicology studies aim to look at the effects of longer-term drug exposure on the body, including repeat dose studies. Pharmaceutical companies deliberately conduct studies over longer time periods than a human would take the drug for, with the duration of the study dictated by the anticipated exposure time that a human would face. For example, if the drug regime calls for daily doses for a seven-day period in humans, animal models undergo the treatment for four weeks. Similarly, a 30-day repeated dose in humans would call for a three-month trial in both animal species.
During these studies, researchers assess parameters such as food and water consumption, body weight, hematology and urine analysis. These aim to monitor for any adverse effects that could arise as a result of taking the drug. Researchers will also monitor immune responses, such as infection occurrences, tumour incidences and histological changes in the immune system.
60% of potential drugs fail in the preclinical phase of pharmaceutical product development.
Analysis And Bioinformatics In The Preclinical Phase
Bioinformatics is core to every stage of the drug development process. For example, data landscaping or mining of available databases or analysis of in-house HTS records can massively help at the R&D stage.
For preclinical development on the other hand, bioinformatics can help give more insight into the method of action of a therapeutic. Toxicology testing provides researchers with context for up- and down-regulated genes – whether the change in regulation could be harmful, whether there are associations that need further investigation. Additionally, they can glean more detailed information at this stage to guide research and phased clinical trials.
Clinical Development
Since you now have an understanding of the preclinical phase of drug development, you may be interested in learning about the clinical side of the drug development process.
Preclinical Phase of the Drug Development Process Video
This video contains a condensed version of the information available in this blog.
Request a sample of our CCLE data analysis & integration reports
The purpose of this sample report is to demonstrate the approach taken by Fios Genomics for the integration of gene expression, copy number, and mutation datasets from the Broad Institute Cancer Cell Line Encyclopaedia (CCLE), with BRAF gene dependency data from the Broad Institute Avana Achilles project. The aim was to identify genetic features that were significantly associated with BRAF gene dependency across the DepMap cancer cell lines and indications. This approach highlighted biomarkers of BRAF dependency and could help prioritise potential novel therapeutic targets.

