Bias in Randomised Trials
- 26th January 2020
- Posted by: Claudine Gabriele
- Category: Articles

Following on from our previous blog on selection bias in randomised trials, we take another look at different types of bias that can occur in trials: detection, attrition, and reporting biases. These are common types of bias that could present during a clinical trial, but it is not an exhaustive list.
Different types of bias in randomised trials
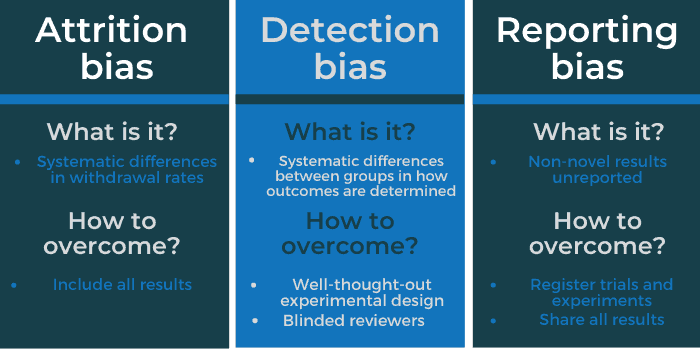
What is detection bias?
Detection bias is the “systematic differences between groups in how outcomes are determined”. It can cause over- or underestimation of the true size of the effect of a treatment, depending on how it has occurred. Detection bias can occur due to a number of reasons.
Detection bias commonly occurs when those who are assessing patients have knowledge of the treatment given. By knowing whether a patient received a certain drug or a placebo, the assessor may or may not be able to “detect” an outcome. On average, non-blinded assessors overestimated odds ratios by 36% – a huge increase.
Secondly, detection bias can be common due to natural variations in a population:
A study of breast cancer survivors showed there were differences in rates of secondary breast cancer between women taking antibiotics, statins and no treatment at all. However, it was also found that there were systematic differences in the number of screenings for secondary cancers that each woman received depending on the medications that had been prescribed to them. The differences in the screening amount could overestimate the risk of secondary breast cancer from taking certain treatments.
In reverse, detection bias could also underestimate the levels of risk. If a man has a larger prostate, diagnosing prostate cancer may be more difficult (there is a lower chance of hitting the target). Obesity increases the risk of prostate cancer, however it is possible to underestimate the association if the size of the prostate is not taken into account.
What is attrition bias?
Another type of bias which can effect randomised trials is attrition bias. Attrition bias is where there are systematic differences in the withdrawal rate between groups of participants in a study. This attrition, where outcome data are not available, can happen for a number of reasons. For example, patients may have been included who are now ineligible, treatments offered were not adhered to, or patients may refuse to participate further in the trial.
Attrition from a trial could also be due to serious side effects or exacerbation of the target illness or disease. If this is the case, removing patients could unduly affect the results and so it is important to include results from all patients who take part the trial.
What is reporting bias?
A common issue throughout science, reporting bias is where researchers fail to report non-novel or significant results. Selective reporting of results can occur as statistically significant differences between groups are more likely to be reported than non-significant ones.
Common types of bias in randomised trials: overview
How to overcome bias in randomised trials
In most cases, good experimental design will reduce bias in randomised trials.
Groups of trial participants should be relatively similar (in terms of ages, genders, etc.). Other factors that could influence results should be evenly spread to avoid differences that could cause discrepancies in results. Taking steps to blind reviewers can also help to mitigate the risk of detection bias. This can be an especially important step where the assessment is of subjective outcomes such as degree of pain.
For reporting bias, registering trials and experiments before they start can help to ensure accountability and follow-up as they progress. The results, whether significant or not, can then be shared. All clinical trials that take place in the European Union (since 2015) have a requirement to publish their results, “regardless of their positive or negative implications”.
How can Fios help?
Fios can help at any stage of your research; from pre-clinical research through to clinical studies. Find out more about some of the areas we work in now, and get in touch through our contact form or email now.
Read more
Biases in randomized trials: a conversation between trialists and epidemiologists
Assessing the quality of controlled clinical trials
Leave a Reply
You must be logged in to post a comment.

