Reducing the Drug Development Timeline
- 24th April 2023
- Posted by: Breige McBride
- Categories: Articles, Bioinformatics

Looking for ways to reduce drug development timelines, or wondering why drug development takes so long? Find the answers you are looking for below! In this blog, we explain each stage of the drug development timeline and how much time they take. Then, we take a look at how drug developers can reduce their timeline for drug development going forward.
Drug Development Timeline
Developing a drug can take anywhere from under 5 years to over 20 years. However, these are the extreme ends of the scale and on average, it takes between 10 and 15 years to develop a new drug1. The exact time taken varies company by company and there are several factors that can influence company drug development timelines. For example, the company’s level of experience in drug development and whether they have had previous drug applications approved can have an impact2.
Another example relates to the demand for the drug being developed. Regulatory agencies like the Food and Drug Administration (FDA) have programmes to speed up development for certain drugs, such as those intended for unmet medical needs. For instance, some drug candidates may receive accelerated approval, as was the case for some drugs developed in response to the COVID-19 pandemic. For a clearer picture of the drug development timeline stage-by-stage, please see our infographic below.
Stages of Drug Development Infographic
There are 8 stages of drug development, and the time each takes varies. The timeline infographic below shows the order of these stages and roughly how long different parts of the drug development journey take.
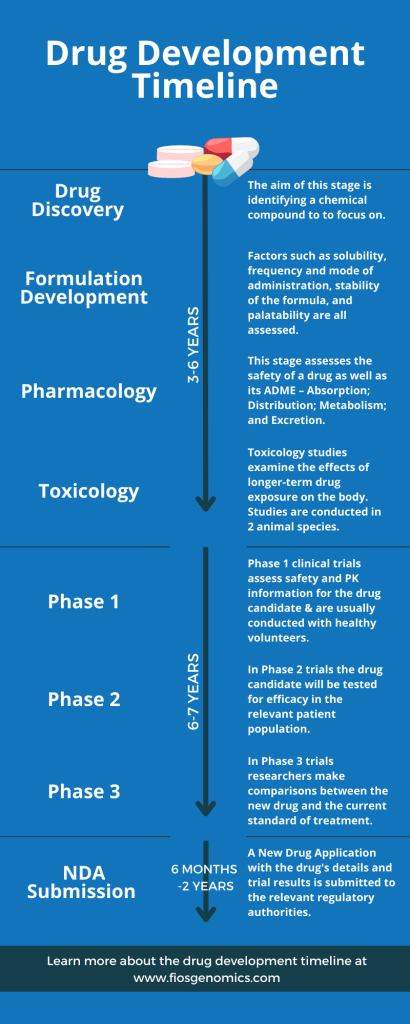
The drug development timeline above gives a brief insight into each stage of the drug development journey. However, to learn in detail what happens at each of these stages you can visit our preclinical drug development and clinical drug development blogs.
Why is drug development so expensive?
The cost of the full process of developing a new drug can be as much as $2.6 billion dollars3. Which is indeed, very expensive. This expense is due to the complexity and time-consuming nature of drug development. Not only are there teams of expert researchers to pay salaries to over a number of years, development also requires a vast array of expensive cutting-edge laboratory instruments, as well as consumables. There are also overhead costs including laboratory space, utilities and administrative costs. In addition to these are the costs for animal models and preclinical studies.
Then, comes the time for phase 1, 2 and 3 clinical trials. Data published in 2016 showed the cost of a phase 3 clinical trial in the United States ranged between $11.5 million to $52.9 million4. All of these costs add up, especially considering that any time a drug candidate fails to pass to the next stage of the drug development journey, it can result in starting the whole process again with a new drug candidate.
How to Reduce the Drug Development Timeline
Since drug development is so expensive, it is vital to reduce overall drug development time wherever possible. There are a few ways to do this. For example, researchers can run processes in tandem wherever possible, particularly in the early stages of development. Another practice which can shave significant time off the drug development timeline, is to focus on repositioning an existing drug. By repositioning an existing drug for a new purpose, pharmaceutical companies can bypass many of the early drug development steps and safety tests, that the drug has already passed. This can take years off of the overall timeline.
Reducing the Drug Development Timeline with Bioinformatics
Another time-saving solution in drug development is to use bioinformatics analyses to their full potential. Bioinformatics analyses are beneficial throughout the drug development process. For example, they can be used in early development to speed up the process of identifying and validating drug targets and leads. For instance, analyses of the data generated from High-Throughput Screens (HTS) can be used to identify a drug lead.
Bioinformatics analyses are also beneficial for analysing clinical trial data to reveal insights to inform next steps. Not only does this save time by cutting down research time between drug development steps, by indicating what the next area to focus on should be, it also helps ensure maximum return on investment by informing decisions and research paths to take that are the most likely to lead to a successful drug candidate. To learn more about how bioinformatics analyses can be beneficial during the drug development process, visit our bioinformatics and the pharmaceutical industry blog.
Alternatively, to discuss how bioinformatics analyses can support your particular drug development project, no matter what stage it is at, contact us today. Our bioinformatics experts are always happy to share their expert advice. They can also demonstrate how bioinformatics analyses can benefit research like yours by sharing example bioinformatics analysis reports.
Author: Breige McBride, Content and Social Media Manager, Fios Genomics
Reviewed by Fios Genomics Bioinformatics Experts to ensure accuracy
Sources
See Also:
Overview of the Preclinical Phase of the Drug Development Process
Overview of the Clinical Phase of the Drug Development Process
The Clinical Trial Diversity Problem
Are You Making the Most of Your Drug Development Data?
Leave a Reply
You must be logged in to post a comment.

